Hormonal Cycles: Fertilization and Early Development
Objectives:
•
explore in detail the events that occur during the ovarian and menstrual cycles
•
describe in detail the process of fertilization followed by the subsequent
development of the conceptus into the pre-embryonic period
The
functions of the female reproductive cycle are to prepare the egg, often
referred to as the gamete or oocyte, for fertilization by the spermatozoon
(sperm), and to prepare the uterus to receive and nourish the fertilized oocyte.
If fertilization has not taken place the inner lining of the uterus or
endometrium and the oocyte are shed and bleeding occurs per vagina, and the
cyclic events begin again.
Before
the onset of puberty, luteinizing hormone (LH) and follicle stimulating hormone
(FSH) levels are low. Pulsatile increases in gonadotrophin releasing hormone (GnRH),
particularly at night, cause increase in LH secretion. This increasing surge of
LH is established prior to menarche (Wennink et al 1990). It is also thought
that the interaction of leptin with GnRH may have a role in the initiation of
puberty. The first-ever occurrence of cyclic events is termed menarche, meaning
the first menstrual bleeding. The average age of menarche is 12 years, although
between the ages 8 and 16 is considered normal. The onset of menstrual bleeding
(‘periods or menses) is a major stage in a girl’s life, representing the
maturation of the reproductive system and physical transition into womanhood.
For many women this monthly phenomenon signals and embodies the quintessence of
being a ‘woman’.
Similarly,
for other women it is regarded as an inconvenience, causing pain, shame and
embarrassment (Chrisler 2011). Cultural and religious traditions affect how
women and their communities feel about menstruation. The advent of hormonal
contraception affords women, especially those in Western society, an element of
control over their periods. Factors such as heredity, diet, obesity and overall
health can accelerate or delay menarche.
Interference
with the hormonal–organ relationship prior to and during the reproductive years
is likely to cause menstrual cycle dysfunction which may result in failure to ovulate.
The cessation of cyclic events is referred to as the menopause, and signifies
the end of reproductive life. Each woman has an individual reproductive cycle
that varies in length, although the average cycle is normally 28 days long, and
recurs regularly from puberty to the menopause except when pregnancy intervene.
THE OVARIAN CYCLE
The
ovarian cycle is the name given to the physiological changes that occur in the
ovaries essential for the preparation and release of an oocyte. The ovarian
cycle consists of three phases, all of which are under the control of hormones.
The follicular phase
The
formation of oogonia in the germinal epithelium of the ovaries is known as
oogenesis. Primordial germ cells differentiate into oogonia in the ovaries
during fetal life. These diploid stem cells divide mitotically and proliferate into
millions of germ cells. Most of the germ cells degenerate (by atresia), however
some develop further into primary oocytes, and enter the prophase
of meiosis I cell division.
Meiotic
arrest occurs and the process does not continue until after puberty (further
meiotic division takes place at ovulation of the secondary oocyte and the
process is only completed if fertilization occurs). Whilst in this arrested prophase
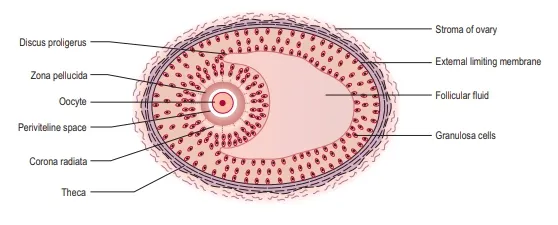
stage of meiosis I the primary oocyte is surrounded by follicular cells and is
hence known as the primordial follicle. There are up to 2 million
primary oocytes in each ovary at birth and due to atresia, the number is reduced
to approximately 40000 at puberty; 400 of these will mature and ovulate during
the woman’s lifetime (Tortora and Derrickson 2011). Following puberty FSH and
LH further stimulate the development of primordial follicles into primary and
secondary follicles and subsequently into large preovulatory or Graafian
follicles by a process known as folliculogenesis.
Low
levels of estrogen and progesterone stimulate the hypothalamus to produce GnRH.
This releasing hormone causes the production of FSH and LH by the anterior pituitary
gland. FSH controls the growth and maturity of the Graafian follicles. The
Graafian follicles begin to secrete estrogen, which comprises estradiol,
estrone and estriol. Rising levels of estradiol cause a surge in LH. When estradiol
reaches a certain peak, the secretion of FSH is inhibited. The reduced FSH
secretion causes a slowing in follicle growth and eventually leads to follicle death,
known as atresia. The largest and dominant follicle secretes inhibin, which
further suppresses FSH. This dominant follicle prevails and forms a bulge near
the surface of the ovary, and soon becomes competent to ovulate. The time from
the growth and maturity of the
Graafian
follicles to ovulation is normally around 1 week. Occasionally the follicular
phase may take longer if the dominant follicle does not ovulate, and the phase
will begin again. The differing lengths of menstrual cycle reported between
individual women are as a result in the varying timespans in this pre-ovulatory
phase. It can last 6–13 days in a 28-day cycle (Tortora and Derrickson 2011).
Ovulation
High
estrogen levels cause a sudden surge in LH around day 12–13 of a 28 day cycle,
which lasts for approximately 48 hours. This matures the oocyte and weakens the
wall of the follicle and causes ovulation to occur on day 14.
Ovulation
is the process whereby the dominant Graafian follicle ruptures and discharges
the secondary oocyte into the pelvic cavity. Fimbrae guide it into the uterine
tube where it awaits fertilization. During the time of ovulation, meiotic cell
division resumes and the diploid oocyte becomes haploid (with a first polar
body). During ovulation some women experience varying degrees of abdominal pain
known as mittelschmerz, which can last several hours. There may be some light
bleeding caused by the hormonal changes taking place. Stringy clear mucus appears
in the cervix, ready to accept the sperm from intercourse. Following ovulation,
the fertilized or unfertilized oocyte travels to the uterus.
The luteal phase
The
luteal phase is the process whereby the cells of the residual ruptured follicle
proliferate and form a yellow irregular structure known as the corpus luteum.
The corpus luteum produces estrogen, relaxin, inhibin and progesterone for approximately
2 weeks, to develop the endometrium of the uterus, which awaits the fertilized oocyte.
Small amounts of relaxin cause uterine quiescence, which is an ideal
environment for the fertilized oocyte to implant. The corpus luteum continues
its role until the placenta is adequately developed to take over. During the luteal
phase the cervical mucus becomes sticky and thick.
In
the absence of fertilization, the corpus luteum degenerates and becomes the
corpus albicans (white body), and progesterone, estrogen, relaxin and inhibin
levels decrease. In response to low levels of estrogen and progesterone the
hypothalamus produces GnRH. The rising levels of GnRH stimulate the anterior
pituitary gland to produce FSH and the ovarian cycle commences again (Stables
and Rankin 2010). The luteal phase is the most constant part of the ovarian
cycle, lasting 14 days out of a 28day cycle (Tortora and Derrickson 2011).
THE MENSTRUAL OR ENDOMETRIAL CYCLE
The
menstrual cycle is the name given to the physiological changes that occur in
the endometrial layer of the uterus, and which are essential to receive the
fertilized oocyte. The menstrual cycle consists of three phases.
The menstrual phase
This
phase is often referred to as menstruation, bleeding, menses, or a period.
Physiologically this is the terminal phase of the reproductive cycle of events
and is simultaneous with the beginning of the follicular phase of the ovarian
cycle. Reducing levels of estrogen and progesterone stimulate prostaglandin release
that causes the spiral arteries of the endometrium to go into spasm,
withdrawing the blood supply to it, and the endometrium dies, referred to as
necrosis. The endometrium is shed down to the basal layer along with blood from
the capillaries, the unfertilized oocyte tissue fluid, mucus and epithelial
cells. Failure to menstruate (amenorrhea) is an indication that a woman may
have become pregnant. The term eumenorrhea denotes normal, regular menstruation
that lasts for typically 3–5 days, although 2–7 days is considered normal.
The
average blood loss during menstruation is 50–150 ml. The blood is inhibited
from clotting due to the enzyme plasmin contained in the endometrium. The
menstrual flow passes from the uterus through the cervix and the vagina to the
exterior. The term menorrhagia denotes heavy bleeding. Some women experience
uterine cramps caused by muscular contractions to expel the tissue. Severe
uterine cramps are known as dysmenorrhea.
The proliferative phase
This
phase follows menstruation, is simultaneous with the follicular phase of the
ovary and lasts until ovulation. There is the formation of a new layer of
endometrium in the uterus, referred to as the proliferative endometrium. This
phase is under the control of estradiol and other estrogens secreted by the
Graafian follicle and consist of the re-growth and thickening of the
endometrium in the uterus. During the first few days of this phase the endometrium
is re-forming, described as the regenerative phase. At the
completion of this phase the endometrium consists of three layers.
The
basal layer lies immediately above the
myometrium and is approximately 1 mm thick. It contains all the
necessary rudimentary structures for building new endometrium.
The
functional layer, which contains tubular
glands, is approximately 2.5 mm thick, and lies on top of the basal
layer. It changes constantly according to the hormonal influences of the
ovary.
The
layer of cuboidal ciliated epithelium
covers the functional layer. It dips down to line the tubular glands of the
functional layer. If fertilization occurs, the fertilized oocyte implants
itself within the endometrium.
The secretory phase
This
phase follows the proliferative phase and is simultaneous with ovulation. It is
under the influence of progesterone and estrogen secreted by the corpus luteum.
The functional layer of the endometrium thickens to approximately 3.5 mm and
becomes spongy in appearance because the glands are more tortuous. The blood
supply to the area is increased and the glands produce nutritive secretions
such as glycogen. These conditions last for approximately 7 days, awaiting the
fertilized oocyte.
FERTILIZATION
Human
fertilization, known as conception, is the fusion of genetic material from the
haploid sperm cell and the secondary oocyte (now haploid), to form the zygote.
The process takes approximately 12–24 hours and normally occurs in the ampulla
of the uterine tube. Following ovulation, the oocyte, which is about 0.15 mm in
diameter, passes into the uterine tube. The oocyte, having no power of
locomotion, is wafted along by the cilia and by the peristaltic muscular
contraction of the uterine tube.
At
the same time the cervix, which is under the influence of estrogen, secretes a
flow of alkaline mucus that attracts the spermatozoa. In the fertile male at
intercourse approximately 300 million sperm are deposited in the posterior fornix
of the vagina. Approximately 2 million reaches the loose cervical mucus,
survive and propel themselves towards the uterine tubes while the rest are
destroyed by the acid medium of the vagina. Approximately 200 sperm will
ultimately reach the oocyte (Tortora and Derrickson 2011). Sperm swim from the
vagina and through the cervical canal using their whip-like tails (flagella).
Prostaglandins from semen and uterine contractions as a result of intercourse
facilitate the passage of the sperm into the uterus and beyond. Once inside the
uterine tubes (within minutes of intercourse), the sperm undergo a process known
as capacitation. This process takes up to 7 hours.
Influenced
by secretions from the uterine tube the sperm undergo changes to the plasma
membrane, resulting in the removal of the glycoprotein coat and increased
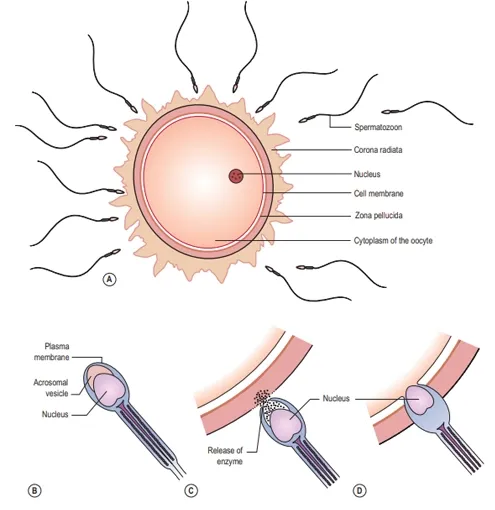
flagellation. The zona pellucida of the oocyte produces chemicals that attract
capacitated sperm only. The acrosomal layer of the capacitated sperm becomes
reactive and releases the enzyme hyaluronidase known as the acrosome reaction, which
disperses the corona radiata (the outermost layer of the oocyte) allowing
access to the zona pellucida. Many sperm are involved in this process. Other enzymes,
such as acrosin, produce an opening in the zona pellucida. The first sperm that
reaches the zona pellucida penetrates it.
Upon
penetration the oocyte releases corticol granules; this is known as the
cortical reaction. The cortical reaction and depolarization of the oocyte cell
membrane makes it impermeable to other sperm. This is important as there are
many sperm surrounding the oocyte at this time. The plasma membranes of the
sperm and oocyte fuse. The oocyte at this stage completes its second meiotic
division, and becomes mature. The pronucleus now has 23 chromosomes, referred
to as haploid. The tail and mitochondria of the sperm degenerate as the sperm
penetrates the oocyte, and there is the formation of the male pronucleus.
The
male and female pronuclei fuse to form a new nucleus that is a combination of
the genetic material from both the sperm and oocyte, referred to as a diploid
cell. The male and the female gametes each contribute half the complement of
chromosomes to make a total of 46. This new cell is called a zygote.
Dizygotic
twins (fraternal twins) are produced from two oocytes released independently
but in the same time frame fusing with two different sperm; they are
genetically different from each other. Monozygotic twins develop from a single
zygote for a variety of reasons, where cells separate into two embryos, usually
before 8 days following fertilization. These twins are genetically identical.
DEVELOPMENT OF THE ZYGOTE
The
development of the zygote can be divided into three periods. The first 2 weeks
after fertilization, referred to as the pre-embryonic period, includes the
implantation of the zygote into the endometrium; weeks 2–8 are known as the
embryonic period; and weeks 8 to birth are known as the fetal period.
The pre-embryonic period
During
the first week the zygote travels along the uterine tube towards the uterus. At
this stage a strong membrane of glycoproteins called the zona pellucida
surrounds the zygote. The zygote receives nourishment, mainly glycogen, from
the goblet cells of the uterine tubes and later the secretory cells of the
uterus. During the travel the zygote undergoes mitotic cellular replication and
division referred to as cleavage, resulting in the formation of smaller cells known
as blastomeres. The zygote divides into two cells at 1 day, then four at
2 days, eight by 2.5 days, 16 by 3 days, now known as the morula. The cells
bind tightly together in a process known as compactation. Next
cavitation occurs whereby the outermost cells secrete fluid into the morula and
a fluid-filled cavity or blastocele appears in the morula.
This
results in the formation of the blastula or blastocyst, comprising 58 cells.
The process from the development of the morula to the development of the
blastocyst is referred to as blastulation and has occurred by around day 4.
The
zona pellucida remains during the process of cleavage, so that despite an
increase in number of cells the overall size remains that of the zygote and
constant at this stage. The zona pellucida prevents the developing blastocyst
from increasing in size and therefore getting stuck in the uterine tube; it
also prevents embedding occurring in the tube rather than the uterus, which
could result in an ectopic pregnancy. Around day 4 the blastocyst enters the uterus.
Endometrial glands secrete glycogen-rich fluid into the uterus which penetrates
the zona pellucida. This and nutrients in the cytoplasm of the blastomeres
provides nourishment for the developing cells. The blastocyst digests its way
out of the zona pellucida once it enters the uterine cavity. The blastocyst
possesses an inner cell mass or embryoblast, and an outer cell mass or
trophoblast. The trophoblast becomes the placenta and chorion, while the embryoblast
becomes the embryo, amnion and umbilical cord (Carlson 2004; Tortora and
Derrickson 2011).
During
week 2, the trophoblast proliferates and differentiates into two layers: the
outer syncytio-trophoblast or syncytium and the inner cytotrophoblast (cuboidal
dividing cells). Implantation of the trophoblast layer into the endometrium,
now known as the decidua, begins. Implantation is usually to the upper
posterior wall. At the implantation stage the zona pellucida will have totally disappeared.
The syncytiotrophoblast layer invades the decidua by forming finger-like
projections called villi that make their way into the decidua and spaces called
lacunae that fill up with the mother’s blood. The villi begin to branch, and
contain blood vessels of the developing embryo, thus allowing gaseous exchange
between the mother and embryo. Implantation is assisted by proteolytic enzymes
secreted by the syncytiotrophoblast cells that erode the decidua and assist
with the nutrition of the embryo. The syncytiotrophoblast cells also produce human
chorionic gonadotrophin (hCG), a hormone that prevents menstruation and
maintains pregnancy by sustaining the function of the corpus luteum.
Simultaneous
to implantation, the embryo continues developing. The cells of the embryoblast
differentiate into two types of cells: the epiblast (closest to the
trophoblasts) and the hypo-blast (closest to the blastocyst cavity). These two
layers of cells form a flat disc known as the bilaminar embryonic disc. A
process of gastrulation turns the bilaminar disc into a tri-laminar embryonic
disc (three layers).
During
gastrulation, cells rearrange themselves and migrate due to predetermined
genetic coding. Three primary germ layers are the main embryonic tissues from which
various structures and organs will develop. The first appearance of these
layers, collectively known as the primitive streak, is around day 15.
•
The ectoderm is the start of tissue that covers most surfaces of the body: the
epidermis layer of the skin, hair and nails. Additionally, it forms the nervous
system.
•
The mesoderm forms the muscle, skeleton, dermis of skin, connective tissue, the
urogenital glands, blood vessels, and blood and lymph cells.
•
The endoderm forms the epithelial lining of the digestive, respiratory and
urinary systems, and
glandular
cells of organs such as the liver and pancreas.
The
epiblast separates from the trophoblast and forms the floor of a cavity, known
as the amniotic cavity. The amnion forms from the cells lining the cavity. The
cavity is filled with fluid, and gradually enlarges and folds around the
bilaminar disc to enclose it. This amniotic cavity fills with fluid (amniotic
fluid) derived initially from maternal filtrate; later the fetus contributes by
excreting urine. Fetal cells can be found in the amniotic fluid and can be used
in diagnostic testing for genetic conditions via a procedure known as
amniocentesis.
At
about 16 days mesodermal cells form a hollow tube in the midline called the
notochordal process; this becomes a more solid structure, the notochord, about
a week later. Specialized inducing cells and responding tissues cause development
of the vertebral bodies and intervertebral discs to occur. The neural tube is
developed from further cell migration, differentiation and folding of embryonic
tissue. This occurs in the middle of the embryo and develops towards each end.
The whole process is known as neurulation. Teratogens, diabetes or folic acid
deficiency may lead to neural tube defects.
The
hypoblast layer of the embryoblast gives rise to extra-embryonic structures
only, such as the yolk sac. Hypoblast cells migrate along the inner
cytotrophoblast lining of the blastocele-secreting extracellular tissue which
becomes the yolk sac. The yolk sac is lined with extraembryonic endoderm, which
in turn is lined with extraembryonic mesoderm. The yolk sac serves as a primary
nutritive function, carrying nutrients and oxygen to the embryo until the
placenta fully takes over this role.
The
endoderm and mesoderm cells contribute to the formation of some organs, such as
the primitive gut arising out of the endoderm cells. An outpouching of
endodermic tissue forms the allantois, this extends to the connecting stalk
around which the umbilical cord later forms. Growth of blood vessels is
induced, connecting separately to vessels of the embryo and placenta (Kay et al
2011). Blood islands that later go on to develop blood cells arise from the
mesodermal layer; the remainder resembles a balloon floating in front of the
embryo until it atrophies by the end of the 6th week when blood-forming activity
transfers to embryonic sites. After birth, all that remains of the yolk sac is
a vestigial structure in the base of the umbilical cord, known as the
vitelline duct.
The
pre-embryonic period is crucial in terms of initiation and maintenance of the
pregnancy and early embryonic development. Inability to implant properly can results
in ectopic pregnancy or miscarriage. Additionally chromosomal defects and
abnormalities in structure and organs can occur during this time (Moore and Persaud
2003).
During
embryological development stem cells under predetermined genetic control become
specialized giving rise to further differentiation with a varying functionality
according to their predefined role.
REFERENCES
1.
Carlson B M 2004 Human embryology and developmental biology, 3rd edn. Mosby,
Philadelphia
2.
Chrisler J C 2011 Leaks, lumps, and lines: stigma and women’s bodies. Psychology
of Women Quarterly 35(2):202–14
3.
Human Tissue Authority (HTA) 2010 guidance for licensed establishments involved
in cord blood collection. Accessed online at www.hta.gov.uk
(11 April 2013)
4.
Kay H H, Nelson D M, Wang Y 2011. The placenta. From development to disease.
Oxford, Wiley–Blackwell Moore K L, Persaud T V N 2003 Before we are born:
essentials of embryology and birth defects, 8th edn. Saunders,
London
5.
Royal College of Obstetricians and Gynaecologists (RCOG)/Royal College of
Midwives (RCM) 2011 Statement on umbilical cord blood collection and banking.
Available at www.rcog.org.uk
(accessed 11 April 2013)
6.
Stables D, Rankin J 2010 Physiology in childbearing: with anatomy and related
biosciences, 3rd edn. Baillière Tindall, Edinburgh
7.
Tortora G J, Derrickson B 2011. Principles of anatomy and physiology.
Maintenance and continuity of the human body, 13th edn. John Wiley
& Sons, Hoboken, NJ
8.
Trotter S 2008 Cord blood banking and its implications for midwifery practice:
time to review the evidence? MIDIRS Midwifery Digest 18(2):159–64
9.
Wennink J M B, Delemarre-van de Waal H A, Schoemaker R et al 1990 Luteinizing
hormone and follicle stimulating hormone secretion patterns in girls throughout
puberty measured using highly sensitive immunoradiometric assays. Clinical Endocrinology
33(3):333–44